1s2 2s2 2p6 3s2 3p4
gasmanvison
Sep 13, 2025 · 6 min read
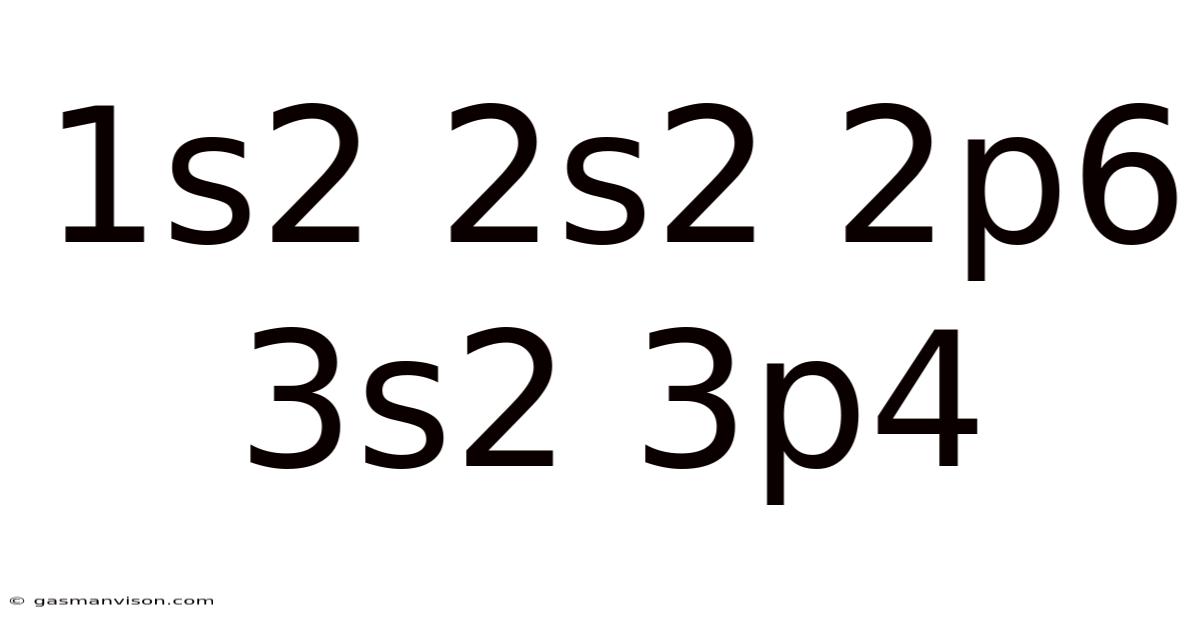
Table of Contents
Decoding 1s² 2s² 2p⁶ 3s² 3p⁴: Unveiling the Secrets of Sulfur's Electronic Structure
This seemingly cryptic string, 1s² 2s² 2p⁶ 3s² 3p⁴, holds the key to understanding the electronic structure of sulfur, a crucial element in numerous biological and industrial processes. This article will delve deep into the meaning behind this notation, exploring its implications for sulfur's chemical properties, reactivity, and overall behavior. We will unpack the underlying principles of electron configuration, orbital filling, and the periodic table's role in predicting these characteristics. By the end, you'll have a firm grasp of how this seemingly simple sequence reveals the complex nature of an atom.
What is Electron Configuration?
Electron configuration describes the arrangement of electrons in the different energy levels and sublevels within an atom. This arrangement dictates how an atom interacts with other atoms, forming chemical bonds and determining its chemical properties. The notation uses numbers and letters to represent these energy levels and sublevels:
- Numbers (1, 2, 3…): These represent the principal energy levels or shells. Electrons closer to the nucleus are in lower energy levels.
- Letters (s, p, d, f): These represent the sublevels within each principal energy level. Each sublevel can hold a specific number of electrons:
- s: Holds a maximum of 2 electrons
- p: Holds a maximum of 6 electrons
- d: Holds a maximum of 10 electrons
- f: Holds a maximum of 14 electrons
- Superscripts (², ⁶, etc.): These indicate the number of electrons in each sublevel.
Understanding Sulfur's Electron Configuration: 1s² 2s² 2p⁶ 3s² 3p⁴
Let's break down sulfur's electron configuration (1s² 2s² 2p⁶ 3s² 3p⁴) step-by-step:
- 1s²: This indicates two electrons in the first energy level (n=1) and the s sublevel. The s sublevel is spherical and closest to the nucleus.
- 2s²: Two electrons are in the second energy level (n=2) and the s sublevel.
- 2p⁶: Six electrons occupy the three 2p orbitals in the second energy level. The p sublevel has three dumbbell-shaped orbitals, each capable of holding two electrons.
- 3s²: Two electrons are found in the 3s orbital of the third energy level (n=3).
- 3p⁴: Four electrons populate the three 3p orbitals in the third energy level.
The Significance of the 3p⁴ Sublevel
The 3p⁴ sublevel is particularly important in determining sulfur's chemical properties. A completely filled p sublevel (containing six electrons) represents a stable configuration. Sulfur, with only four electrons in its 3p sublevel, is one step away from achieving this stable octet (eight electrons in its outermost shell). This incomplete octet is the driving force behind sulfur's reactivity. To achieve stability, sulfur readily gains two electrons, forming the sulfide anion (S²⁻), or it shares electrons to form covalent bonds.
Sulfur's Chemical Properties and Reactivity
Sulfur's electron configuration directly influences its chemical behavior. The tendency to gain two electrons or share electrons to achieve a stable octet results in several key properties:
-
Oxidation States: Sulfur exhibits various oxidation states, reflecting its ability to gain or lose electrons. Common oxidation states include -2 (sulfide), +4 (sulfur dioxide), and +6 (sulfuric acid). This versatility makes sulfur essential in various chemical reactions.
-
Covalent Bonding: Sulfur readily forms covalent bonds with other nonmetals, such as oxygen, hydrogen, and carbon. This ability is evident in molecules like hydrogen sulfide (H₂S), sulfur dioxide (SO₂), and sulfuric acid (H₂SO₄). These covalent bonds are formed by sharing electrons to achieve a stable octet.
-
Ionization Energy: Sulfur's ionization energy, the energy required to remove an electron, is relatively low compared to other nonmetals in its period. This means it's relatively easy to remove electrons from sulfur, particularly from its outermost shell, explaining its reactivity.
-
Electronegativity: Sulfur's electronegativity, its ability to attract electrons in a chemical bond, is relatively high. This contributes to its ability to form polar covalent bonds with more electronegative atoms such as oxygen.
Applications of Sulfur and its Compounds
The unique chemical properties of sulfur derived from its electronic structure make it a vital element with numerous applications:
-
Sulfuric Acid (H₂SO₄): This is arguably sulfur's most important compound, widely used in the production of fertilizers, detergents, and various other chemicals. Its strong acidic nature makes it a crucial industrial chemical.
-
Sulfur Dioxide (SO₂): While a significant air pollutant, sulfur dioxide also finds use in the production of sulfuric acid and as a preservative in food and wine.
-
Hydrogen Sulfide (H₂S): This gas is used in the synthesis of various sulfur-containing compounds and has applications in the petrochemical industry.
-
Vulcanization of Rubber: The addition of sulfur to rubber is a crucial process that increases its strength and durability. This process relies on the chemical reactions of sulfur with the rubber molecules.
Sulfur in Biological Systems
Sulfur plays a critical role in biological systems:
-
Amino Acids: Two essential amino acids, cysteine and methionine, contain sulfur atoms in their structure. These amino acids are essential building blocks of proteins.
-
Enzymes: Many enzymes, which catalyze biochemical reactions, contain sulfur atoms, particularly in cysteine residues. These sulfur atoms contribute to the enzyme's catalytic activity.
-
Iron-Sulfur Clusters: These clusters are vital components of various proteins involved in electron transport and other crucial biological processes. Sulfur atoms form essential bridges within these clusters.
Relating Electron Configuration to the Periodic Table
The periodic table is a powerful tool for predicting the electron configuration of elements. Sulfur's position in the periodic table (Group 16, Period 3) helps us understand its electron configuration:
-
Group 16 (Chalcogens): Elements in this group have six valence electrons (electrons in the outermost shell). This corresponds to the 3s² 3p⁴ configuration in sulfur.
-
Period 3: This indicates that the outermost electrons are in the third energy level (n=3).
Advanced Concepts: Orbital Diagrams and Hund's Rule
While the electron configuration notation (1s² 2s² 2p⁶ 3s² 3p⁴) provides a concise summary of electron arrangement, a more detailed picture emerges using orbital diagrams. These diagrams illustrate how electrons fill individual orbitals within sublevels. Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and leads to a more stable configuration. For sulfur's 3p subshell, this means each of the three 3p orbitals will contain one electron before any orbital receives a second electron.
Conclusion: The Power of Understanding Electronic Structure
The seemingly simple notation, 1s² 2s² 2p⁶ 3s² 3p⁴, reveals a wealth of information about sulfur's electronic structure. This understanding is crucial for predicting its chemical properties, reactivity, and its diverse applications in various fields. By mastering the principles of electron configuration and the periodic table, we gain a powerful tool for understanding the behavior of elements and the complexities of the chemical world. The seemingly simple arrangement of electrons within the sulfur atom dictates its crucial roles in everything from industrial processes to the functioning of life itself. Further exploration into quantum mechanics and advanced chemical principles would build upon this foundational understanding. The connection between seemingly abstract concepts like electron configuration and the tangible properties and applications of elements like sulfur highlights the elegance and power of scientific understanding.
Latest Posts
Latest Posts
-
40 Fl Oz To Ml
Sep 13, 2025
-
Gcf Of 12 And 8
Sep 13, 2025
-
38 Degrees F To C
Sep 13, 2025
-
52 Thousandths In Scientific Notation
Sep 13, 2025
-
How Many Inches Is 7ft
Sep 13, 2025
Related Post
Thank you for visiting our website which covers about 1s2 2s2 2p6 3s2 3p4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.