3r 4s 3 4 Dimethylhexane
gasmanvison
Sep 14, 2025 · 6 min read
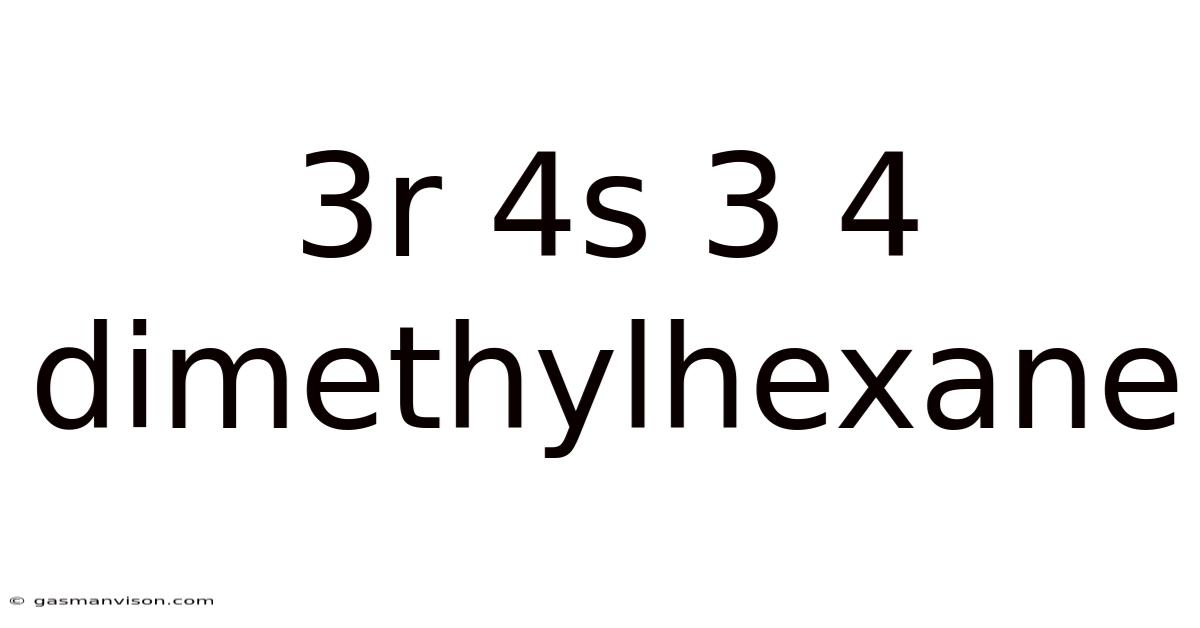
Table of Contents
3R,4S-3,4-Dimethylhexane: A Deep Dive into Stereochemistry and Properties
This article explores the fascinating world of 3R,4S-3,4-dimethylhexane, a chiral molecule with unique stereochemical properties and characteristics. We'll delve into its structure, nomenclature, synthesis, physical properties, and potential applications. Understanding this specific isomer is crucial for grasping fundamental concepts in organic chemistry, particularly stereochemistry and its implications in various fields. This in-depth analysis will also touch upon related compounds and their comparative properties, enhancing your understanding of alkane isomerism.
Meta Description: This comprehensive guide explores 3R,4S-3,4-dimethylhexane, detailing its stereochemistry, nomenclature, synthesis, physical properties, and potential applications. Learn about chiral molecules, alkane isomerism, and the importance of understanding 3D molecular structures.
Understanding the Nomenclature: Deciphering 3R,4S-3,4-Dimethylhexane
The name itself reveals crucial information about the molecule's structure. Let's break it down:
- Hexane: Indicates a six-carbon alkane chain (C₆H₁₄) as the parent structure. This is the foundational backbone of the molecule.
- 3,4-Dimethyl: This signifies two methyl groups (CH₃) attached to the third and fourth carbon atoms of the hexane chain.
- 3R,4S: These prefixes denote the stereochemistry at the chiral centers. The 3 and 4 carbons are chiral centers because each is bonded to four different groups. The 'R' and 'S' designations are based on the Cahn-Ingold-Prelog (CIP) priority rules, a system used to assign absolute configurations to chiral molecules. This indicates the specific spatial arrangement of the atoms around these chiral centers, differentiating this molecule from its numerous stereoisomers.
The CIP rules are based on assigning priorities to the four substituents around the chiral carbon based on atomic number. The molecule is then oriented so that the lowest priority group points away from the viewer. If the priority order of the remaining three groups (highest to lowest) proceeds clockwise, it's designated as 'R' (rectus, Latin for right). If it proceeds counterclockwise, it's designated as 'S' (sinister, Latin for left). Therefore, 3R,4S indicates a specific spatial arrangement of the methyl groups and other substituents.
Isomerism and Chirality: Exploring the Different Forms of 3,4-Dimethylhexane
3,4-Dimethylhexane exhibits various forms of isomerism:
- Constitutional Isomerism: This refers to isomers with the same molecular formula but different connectivity of atoms. 3,4-Dimethylhexane is just one of many constitutional isomers of hexane. Other isomers might have the methyl groups on different carbon atoms, leading to significant variations in their properties.
- Stereoisomerism: This type of isomerism deals with molecules having the same connectivity but different spatial arrangements of atoms. 3,4-Dimethylhexane demonstrates stereoisomerism due to its chiral centers.
- Enantiomerism: 3R,4S-3,4-dimethylhexane is a chiral molecule, meaning it is not superimposable on its mirror image. Its mirror image, 3S,4R-3,4-dimethylhexane, is its enantiomer. Enantiomers have identical physical properties except for their interaction with plane-polarized light and their behavior in chiral environments.
- Diastereomerism: Other stereoisomers of 3,4-dimethylhexane, such as 3R,4R and 3S,4S, are diastereomers. Diastereomers are stereoisomers that are not mirror images of each other. They possess different physical and chemical properties.
Synthesis of 3R,4S-3,4-Dimethylhexane: A Challenging Task
Synthesizing a specific stereoisomer like 3R,4S-3,4-dimethylhexane requires meticulous control over reaction conditions and often involves multiple steps. A straightforward synthesis is challenging due to the need for stereoselective reactions. The synthesis might involve:
- Starting Materials: Careful selection of starting materials is crucial to control the stereochemistry of the final product. This could involve using chiral reagents or catalysts.
- Stereoselective Reactions: Reactions that preferentially form one stereoisomer over others are essential. Examples include asymmetric hydrogenation or stereospecific additions to alkenes.
- Purification: Once synthesized, the 3R,4S-3,4-dimethylhexane must be purified to remove any other isomers or impurities. Techniques such as chromatography might be used for effective separation.
The specific synthetic pathway would require detailed consideration of reaction mechanisms, protecting groups, and reaction yields. It's a complex undertaking requiring specialized knowledge in organic synthesis.
Physical Properties: Examining the Characteristics of 3R,4S-3,4-Dimethylhexane
While precise data for 3R,4S-3,4-dimethylhexane may be limited in publicly accessible databases, we can extrapolate its properties based on its structure and comparison to similar alkanes:
- Boiling Point: It would likely have a boiling point similar to other hexane isomers, influenced by its molecular weight and intermolecular forces (London Dispersion Forces).
- Melting Point: The melting point is sensitive to the packing efficiency in the solid state, influenced by the 3D arrangement of atoms. Specific stereochemistry significantly influences crystal packing, and thus the melting point. Comparison to similar chiral molecules is needed to predict the exact value.
- Density: Similar to boiling point, the density would be similar to that of other hexane isomers, falling within the typical range of alkanes.
- Solubility: As an alkane, it's expected to be insoluble in polar solvents like water but soluble in non-polar organic solvents.
- Optical Rotation: As a chiral molecule, 3R,4S-3,4-dimethylhexane will exhibit optical activity, rotating the plane of plane-polarized light. The specific rotation depends on the concentration, solvent, and temperature. Its enantiomer, 3S,4R-3,4-dimethylhexane, will rotate the plane of polarized light in the opposite direction.
Potential Applications: Exploring the Uses of 3R,4S-3,4-Dimethylhexane
The applications of 3R,4S-3,4-dimethylhexane are likely limited due to its relatively simple structure and lack of significant functional groups. However, its importance lies primarily in its role as a model compound for studying stereochemistry. Its use in research and education is significant:
- Stereochemistry Studies: Its chiral nature makes it invaluable for teaching and research in stereochemistry, illustrating concepts such as enantiomers, diastereomers, and the CIP rules.
- Chiral Recognition: Its distinct stereochemistry can be used to probe the chiral recognition capabilities of various enzymes, receptors, or catalysts.
- Developing Synthetic Methods: Its synthesis could serve as a test bed for developing new stereoselective synthetic methodologies.
Comparison with Other Isomers: Understanding the Differences
Comparing 3R,4S-3,4-dimethylhexane with other isomers, especially its enantiomer (3S,4R) and diastereomers (3R,4R and 3S,4S), highlights the significance of stereochemistry. While the constitutional isomers have significantly different physical and chemical properties due to different connectivities, even subtle changes in the stereochemistry lead to differing interactions with chiral environments. The enantiomers possess identical physical properties in achiral environments, but differ profoundly in their optical activity and behavior in chiral environments such as interactions with enzymes. Diastereomers show differences in both their physical and chemical properties.
Conclusion: The Significance of 3R,4S-3,4-Dimethylhexane
3R,4S-3,4-dimethylhexane, despite its seemingly simple structure, serves as a powerful example to illustrate the importance of stereochemistry in organic chemistry. Understanding its nomenclature, synthesis, physical properties, and relationship to its isomers provides a strong foundation for understanding more complex chiral molecules and their applications in various fields. While its direct industrial applications might be limited, its significance lies in its educational and research value, contributing significantly to the advancement of organic chemistry and our understanding of molecular structure and function. Further research and exploration of its properties, particularly in the context of chiral recognition and catalysis, could unveil potential applications in the future. This detailed analysis emphasizes the intricate world of stereochemistry and the profound impact even seemingly subtle structural differences can have on molecular behavior.
Latest Posts
Latest Posts
-
Homework 8 Equations Of Circles
Sep 14, 2025
-
32 5 Percent As A Decimal
Sep 14, 2025
-
Nature Is To Nurture As
Sep 14, 2025
-
Which Best Describes Traditional Classification
Sep 14, 2025
-
How Many Ml In Ul
Sep 14, 2025
Related Post
Thank you for visiting our website which covers about 3r 4s 3 4 Dimethylhexane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.