Air Is A ___________-____________ Solution.
gasmanvison
Sep 23, 2025 · 7 min read
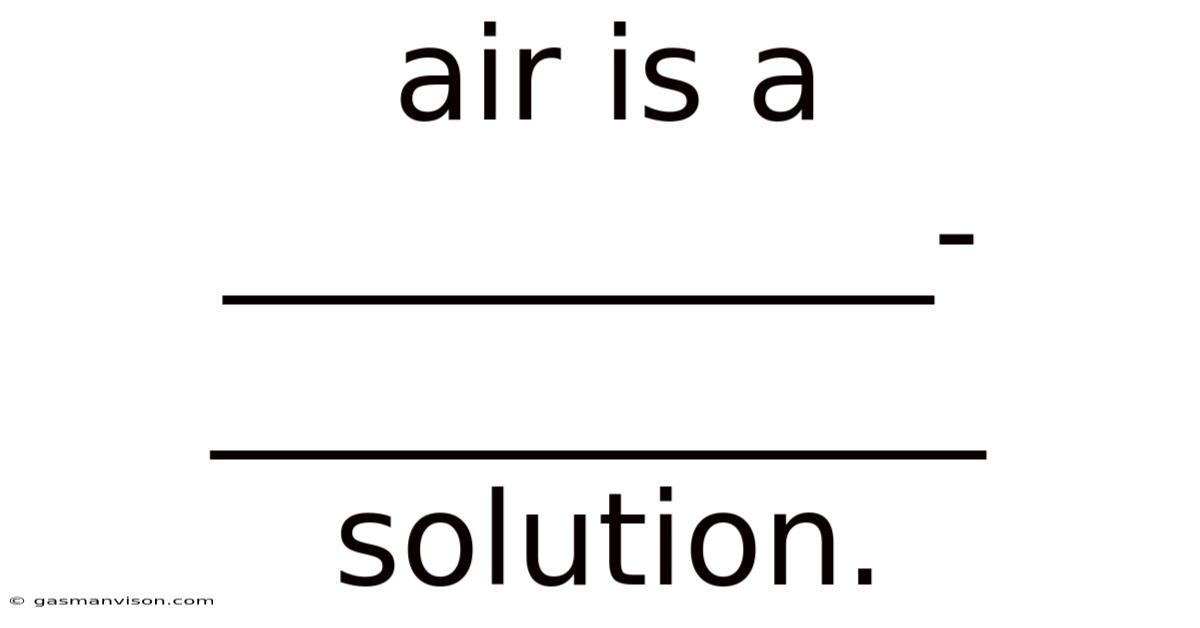
Table of Contents
Air is a Homogeneous Mixture: Delving into the Composition and Properties of Earth's Atmosphere
Air, the invisible lifeblood of our planet, is often taken for granted. We breathe it without a second thought, yet its composition and properties are remarkably complex and crucial to understanding our world. This article will explore the fascinating nature of air, clarifying its classification as a homogeneous mixture, and examining the implications of this characteristic on various aspects of our environment and lives. Understanding air's true nature – a perfectly blended combination of gases – allows us to appreciate its vital role in everything from weather patterns to the very processes that sustain life.
What is a Homogeneous Mixture?
Before diving into the specifics of air, let's define the term "homogeneous mixture." A homogeneous mixture is a type of mixture where the components are uniformly distributed throughout the mixture. This means that at a macroscopic level (meaning visible to the naked eye or with simple magnification), the mixture appears uniform in composition. Unlike heterogeneous mixtures, where different components are visibly distinguishable (like sand and water), in a homogeneous mixture, the individual components are not easily separated visually or by simple physical methods. Examples of homogeneous mixtures include saltwater, air, and many metal alloys.
Air: A Closer Look at its Composition
The air we breathe is primarily a mixture of gases, each contributing in varying proportions to its overall characteristics. The major components are:
-
Nitrogen (N₂): Approximately 78% of Earth's atmosphere is composed of nitrogen. This relatively inert gas plays a vital role in maintaining the stability of the atmosphere and is essential for the growth of plants, though not directly absorbed in its gaseous form. Nitrogen fixation, a process carried out by certain bacteria, is crucial for converting atmospheric nitrogen into usable forms for plants.
-
Oxygen (O₂): Making up roughly 21% of the atmosphere, oxygen is the life-sustaining gas vital for the respiration of most living organisms. Its presence enables cellular respiration, the process that converts food into energy. Oxygen is also highly reactive, participating in combustion and many other chemical processes.
-
Argon (Ar): This inert noble gas constitutes about 0.93% of the atmosphere. Its chemical inertness makes it relatively unreactive and therefore has minimal impact on most atmospheric processes.
-
Other Gases: The remaining fraction of the atmosphere (less than 1%) includes trace gases such as carbon dioxide (CO₂), neon (Ne), helium (He), methane (CH₄), krypton (Kr), hydrogen (H₂), and xenon (Xe). While present in smaller amounts, these gases play significant roles in various atmospheric phenomena. For example, carbon dioxide is a crucial greenhouse gas influencing Earth's climate, while methane is a potent greenhouse gas with a higher global warming potential than carbon dioxide.
The Homogeneity of Air: A Matter of Scale
The statement that air is a homogeneous mixture is accurate at a macroscopic scale. If you were to take a sample of air from one location and compare it to a sample from another location (excluding localized pollutants), the composition would be remarkably similar. The proportions of nitrogen, oxygen, and other gases would be consistent across vast distances, demonstrating its uniform distribution.
However, it’s crucial to acknowledge that at a microscopic level, air is not perfectly uniform. The gas molecules are in constant, random motion, colliding with each other and with other particles. The density of air molecules can fluctuate slightly depending on various factors such as altitude, temperature, and pressure. But these microscopic variations are insignificant in comparison to the overall homogeneity of the mixture.
Factors Affecting the Composition of Air
While air is generally homogeneous, its composition can be locally affected by several factors:
-
Altitude: The composition of air varies with altitude. The concentration of gases like oxygen decreases with increasing altitude due to gravity. The upper layers of the atmosphere are significantly less dense and have different proportions of gases compared to the air we breathe at sea level.
-
Pollution: Human activities release various pollutants into the atmosphere, including particulate matter, sulfur oxides, nitrogen oxides, and volatile organic compounds. These pollutants can significantly alter the local composition of air, particularly in urban areas and industrial regions. This localized inhomogeneity is a significant environmental concern, impacting air quality and human health.
-
Geographic Location: The composition of air can vary slightly depending on geographic location. For example, areas with significant vegetation might have slightly higher levels of oxygen due to photosynthesis. Coastal regions can have a higher concentration of water vapor.
Implications of Air's Homogeneous Nature
The homogeneous nature of air has far-reaching implications:
-
Efficient Gas Exchange: The uniform distribution of oxygen and other gases facilitates efficient gas exchange in the lungs and throughout the body. This homogeneity ensures that every breath provides a relatively consistent supply of oxygen necessary for respiration.
-
Weather Patterns: The homogeneous nature of air, despite variations at different altitudes, is fundamental to understanding large-scale weather patterns. The movement and mixing of air masses are governed by factors such as temperature gradients, pressure differences, and the overall composition of the air.
-
Atmospheric Stability: The relatively uniform composition of air contributes to the stability of the Earth's atmosphere. The consistent proportions of gases create a balanced system that allows for predictable weather patterns and prevents extreme fluctuations in atmospheric conditions.
-
Climate Regulation: The balance of greenhouse gases, while subject to change due to human activities, contributes to the regulation of Earth's climate. The concentrations of gases such as carbon dioxide, methane, and water vapor influence the planet’s temperature and energy balance.
Air as a Solution: A Closer Look
While the term "solution" is generally used to describe homogeneous mixtures of a solute dissolved in a solvent, air can be considered a solution in a broader sense. In this case, nitrogen can be considered the solvent, with oxygen and other gases acting as solutes. This is a convenient way to understand the interaction and behavior of gases in the atmosphere. However, it's important to note that the "solvent-solute" analogy is not as precise for gaseous mixtures as it is for liquid solutions. The interactions between gas molecules are different from those in liquid solutions.
Understanding Air's Properties
Several properties of air are directly linked to its homogeneous nature:
-
Density: Air has a relatively low density compared to other substances. This density is influenced by the combined densities of its constituent gases, which are relatively low.
-
Compressibility: Air is compressible; its volume can be reduced by applying pressure. This is because the gas molecules are widely spaced and can be pushed closer together.
-
Expansibility: Air also expands when heated, which is important for understanding atmospheric dynamics and the formation of weather patterns.
-
Pressure: Atmospheric pressure is the result of the weight of the air column above a given point. The homogeneous distribution of air, at least on a macroscopic scale, creates a relatively even pressure distribution across large areas.
Conclusion: The Vital Role of Air's Homogeneous Nature
The fact that air is a homogeneous mixture is not merely a scientific detail; it is fundamental to our understanding of the atmosphere and its vital role in supporting life on Earth. The consistent composition and distribution of gases facilitate efficient gas exchange, influence weather patterns, regulate climate, and contribute to the overall stability of our environment. While local variations due to pollution and altitude exist, the fundamentally homogeneous nature of air remains a cornerstone of our planet's atmospheric system. Further understanding of this aspect is crucial for addressing challenges such as air pollution and climate change, ensuring the continued health and well-being of our planet and its inhabitants. By appreciating the complexity and delicate balance inherent in Earth's atmosphere, we can work towards preserving this invaluable resource for future generations.
Latest Posts
Latest Posts
-
3 4 Cup Butter In Sticks
Sep 23, 2025
-
What Makes Orange And Blue
Sep 23, 2025
-
150 Degrees Celsius To Fahrenheit
Sep 23, 2025
-
Is 90 An Even Number
Sep 23, 2025
-
Magnesium Hydroxide Acetic Acid
Sep 23, 2025
Related Post
Thank you for visiting our website which covers about Air Is A ___________-____________ Solution. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.