C3h5 No3 3 Molar Mass
gasmanvison
Sep 10, 2025 · 5 min read
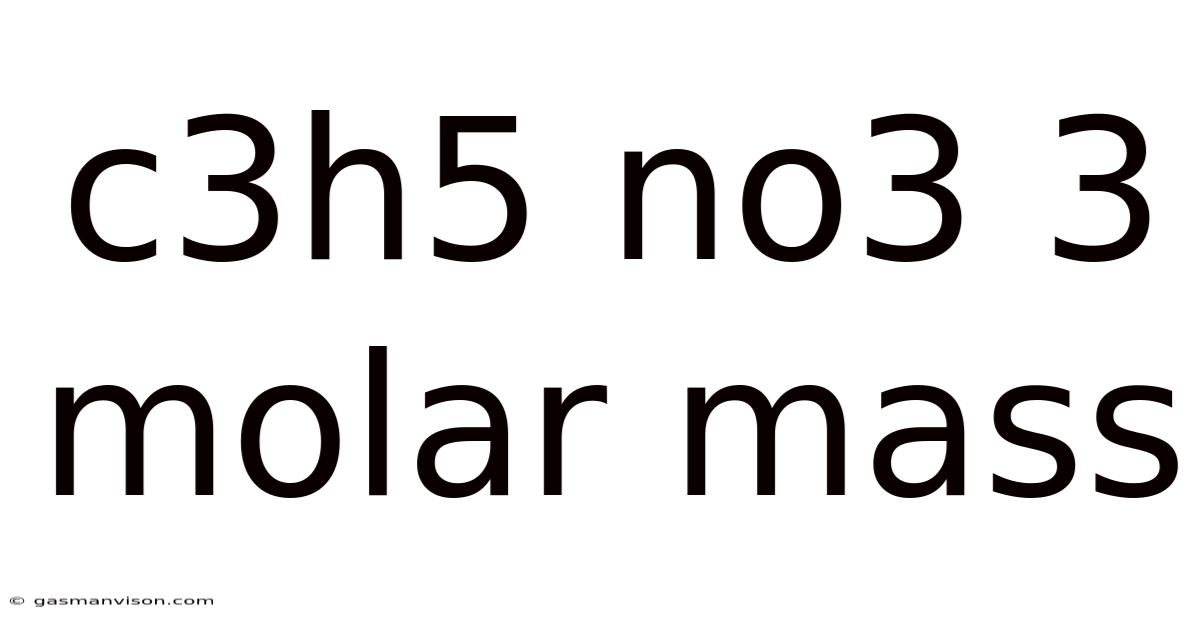
Table of Contents
Decoding the Molar Mass of C3H5(NO3)3: Nitroglycerin's Molecular Weight and its Significance
Nitroglycerin, with its chemical formula C₃H₅(NO₃)₃, is a well-known explosive compound, but its significance extends far beyond its destructive potential. Understanding its molar mass is crucial for various applications, from manufacturing explosives to its use in medicine as a vasodilator. This comprehensive article delves into the calculation of nitroglycerin's molar mass, explores its applications, and touches upon the safety considerations involved in handling this potent chemical.
What is Molar Mass?
Before diving into the calculation for C₃H₅(NO₃)₃, let's establish a clear understanding of molar mass. Molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry representing Avogadro's number (approximately 6.022 x 10²³) of particles (atoms, molecules, ions, etc.). The molar mass is expressed in grams per mole (g/mol). It's essentially a conversion factor that allows us to relate the mass of a substance to the number of molecules present.
Calculating the Molar Mass of C₃H₅(NO₃)₃ (Nitroglycerin)
To calculate the molar mass of nitroglycerin, C₃H₅(NO₃)₃, we need the atomic masses of each element present in the molecule:
- Carbon (C): Approximately 12.01 g/mol
- Hydrogen (H): Approximately 1.01 g/mol
- Nitrogen (N): Approximately 14.01 g/mol
- Oxygen (O): Approximately 16.00 g/mol
Now, let's break down the calculation:
- Carbon (C): 3 carbon atoms x 12.01 g/mol/atom = 36.03 g/mol
- Hydrogen (H): 5 hydrogen atoms x 1.01 g/mol/atom = 5.05 g/mol
- Nitrogen (N): 3 nitrogen atoms x 14.01 g/mol/atom = 42.03 g/mol
- Oxygen (O): 9 oxygen atoms x 16.00 g/mol/atom = 144.00 g/mol
Total Molar Mass: 36.03 g/mol + 5.05 g/mol + 42.03 g/mol + 144.00 g/mol = 227.11 g/mol
Therefore, the molar mass of nitroglycerin, C₃H₅(NO₃)₃, is approximately 227.11 g/mol. This value is crucial for various stoichiometric calculations involving nitroglycerin.
Applications of Nitroglycerin and the Importance of Molar Mass
The molar mass of nitroglycerin is a cornerstone in understanding and utilizing this powerful compound in various fields:
1. Explosives Manufacturing:
Accurate determination of the molar mass is critical for precisely controlling the stoichiometry of nitroglycerin production. This ensures the consistent production of high-quality explosives with predictable explosive properties. The molar mass is used in calculations to determine the amount of reactants needed to synthesize a specific quantity of nitroglycerin. Deviation from the correct molar mass can lead to inconsistencies in the final product, potentially impacting its safety and effectiveness.
2. Pharmaceutical Applications:
Nitroglycerin is a well-established vasodilator used to treat angina pectoris (chest pain). Its precise dosage is crucial, and molar mass calculations are essential for accurate formulation of nitroglycerin tablets, patches, and sprays. Pharmaceutical companies rely on the precise molar mass to determine the correct amount of the active ingredient needed for therapeutic effectiveness, while avoiding harmful overdoses. This is where understanding the molecular weight is crucial for precise drug development and safe medicinal use.
3. Research and Development:
The molar mass is essential for researchers investigating the chemical and physical properties of nitroglycerin. It's a key parameter used in various experiments, simulations, and analytical techniques. Understanding the molar mass is fundamental in analyzing its reactivity, stability, and decomposition mechanisms, as well as developing safer handling protocols.
4. Environmental Monitoring:
In cases of accidental release or contamination, knowing the molar mass of nitroglycerin is essential for accurate environmental monitoring and remediation efforts. It enables scientists to calculate the concentration of nitroglycerin in water, soil, or air samples, facilitating the assessment of environmental impact and the design of effective cleanup strategies.
Safety Considerations when Handling Nitroglycerin
Nitroglycerin is an extremely sensitive explosive compound. Even minor shocks, friction, or heat can cause it to detonate. Its handling requires strict adherence to safety protocols:
- Specialized Training: Only trained personnel should handle nitroglycerin. Thorough training on proper handling procedures, safety equipment, and emergency response is paramount.
- Controlled Environment: Nitroglycerin should be stored and handled in a controlled environment, free from any sources of ignition, friction, or impact.
- Protective Equipment: Appropriate personal protective equipment (PPE) including gloves, eye protection, and specialized clothing must always be worn.
- Proper Storage: Nitroglycerin should be stored in specifically designed containers and facilities that prevent accidental detonation. Temperature and humidity should be closely monitored and controlled to ensure stability.
- Emergency Preparedness: A comprehensive emergency response plan should be in place, including evacuation procedures and the availability of appropriate safety equipment and trained personnel.
Conclusion
The molar mass of C₃H₅(NO₃)₃ (nitroglycerin), approximately 227.11 g/mol, is a fundamental parameter in understanding and utilizing this significant compound. From its applications in explosives manufacturing to its crucial role in medicine, precise calculations involving its molar mass are essential for various aspects of its production, handling, and application. However, it's crucial to always remember that nitroglycerin is a highly sensitive explosive and should be handled only by trained professionals with strict adherence to safety protocols. Understanding its properties and the significance of its molar mass is crucial for both safe and effective utilization. Further research into its properties continues to provide valuable insights, leading to advancements in its applications and safer handling techniques. The accurate calculation of its molar mass remains a critical aspect of this ongoing research. The importance of precise measurements and rigorous safety practices cannot be overstated.
Latest Posts
Latest Posts
-
Assuming You Tested An Organism
Sep 10, 2025
-
What Is 4 11 In Inches
Sep 10, 2025
-
17c Is What In Fahrenheit
Sep 10, 2025
-
Storing Homemade Vinegar In Plastic
Sep 10, 2025
-
Lewis Dot Structure For Of2
Sep 10, 2025
Related Post
Thank you for visiting our website which covers about C3h5 No3 3 Molar Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.