Ch3ch Nh2 Ch2ch Ch3 Oh
gasmanvison
Sep 25, 2025 · 6 min read
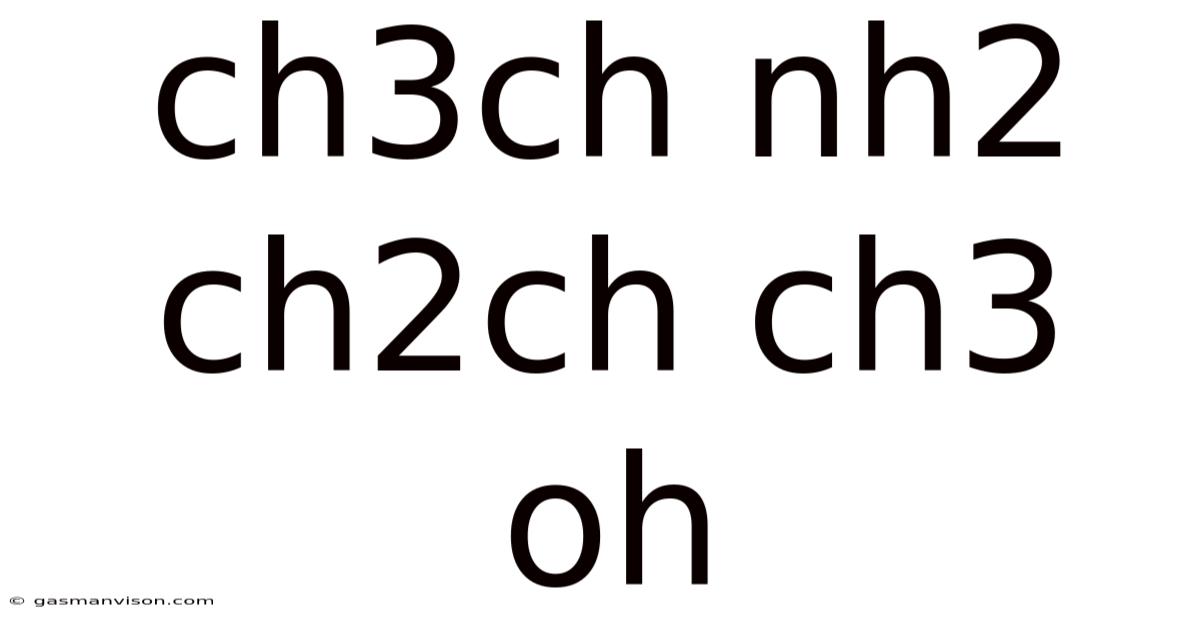
Table of Contents
Unveiling the Chemistry of 2-Amino-4-methylpentan-2-ol: Structure, Properties, and Potential Applications
Meta Description: Delve into the fascinating world of 2-Amino-4-methylpentan-2-ol (a chiral amino alcohol), exploring its chemical structure, unique properties, potential applications, synthesis routes, and safety considerations. This comprehensive guide provides a detailed overview for chemists, students, and anyone interested in organic chemistry.
The chemical formula CH₃CH(NH₂)CH₂CH(CH₃)OH represents 2-Amino-4-methylpentan-2-ol, a chiral molecule belonging to the class of amino alcohols. This compound, often abbreviated as AMP2ol, possesses a unique combination of functional groups – an amino group (-NH₂) and a hydroxyl group (-OH) – attached to a branched carbon chain, leading to interesting chemical properties and potential applications. This article will explore the structure, properties, potential uses, synthesis, and safety considerations associated with this fascinating molecule.
Understanding the Structure and Isomerism of 2-Amino-4-methylpentan-2-ol
The structural formula reveals a carbon chain of five carbons (pentan-), with a methyl group (-CH₃) attached to the fourth carbon atom (4-methyl-), a hydroxyl group (-OH) attached to the second carbon atom (2-ol), and an amino group (-NH₂) also attached to the second carbon atom (2-amino-). This specific arrangement results in a chiral center at the second carbon atom, meaning the molecule exists as two enantiomers – non-superimposable mirror images. These enantiomers, often designated as (R)-2-Amino-4-methylpentan-2-ol and (S)-2-Amino-4-methylpentan-2-ol, exhibit different physical and potentially biological properties. The presence of these chiral centers significantly impacts the molecule's interactions with other chiral molecules, a crucial factor in various applications, including pharmaceuticals and asymmetric catalysis. Understanding the stereochemistry of this compound is fundamental for exploring its potential.
Conformational Analysis and Molecular Dynamics
The flexibility of the carbon chain allows for various conformations. Computational methods, such as molecular mechanics and molecular dynamics simulations, can predict the preferred conformations of AMP2ol in different environments. These studies can provide valuable insights into the molecule's behavior, including its reactivity, interactions with solvents, and potential interactions with biological targets. Factors such as steric hindrance between the substituents influence the energetically favorable conformations. These simulations can be used to predict the behavior of AMP2ol in different applications.
Physicochemical Properties of 2-Amino-4-methylpentan-2-ol
The physicochemical properties of AMP2ol are influenced by the presence of both the amino and hydroxyl functional groups, as well as the branched carbon chain. These properties include:
-
Solubility: The presence of both polar hydroxyl and amino groups contributes to its solubility in polar solvents such as water and alcohols. However, the hydrophobic nature of the alkyl chain might limit its solubility in non-polar solvents. Detailed solubility studies across a range of solvents would provide more quantitative data.
-
Melting Point and Boiling Point: These properties are difficult to predict precisely without experimental data, but the presence of hydrogen bonding between molecules would suggest relatively high melting and boiling points compared to similar non-polar compounds.
-
Acidity and Basicity: The amino group exhibits basic properties, capable of accepting a proton (H⁺), while the hydroxyl group can act as a weak acid, donating a proton. The pKa values for both functional groups are important parameters for understanding its behavior in different pH environments.
-
Optical Rotation: As a chiral molecule, AMP2ol exhibits optical activity, meaning it rotates the plane of polarized light. The specific rotation depends on the enantiomeric purity and the wavelength of light used.
-
Spectroscopic Properties: Techniques such as nuclear magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy, and mass spectrometry (MS) can be employed to characterize AMP2ol and confirm its structure. Specific NMR chemical shifts, IR absorption bands, and mass-to-charge ratios provide crucial structural information.
Potential Applications of 2-Amino-4-methylpentan-2-ol
The unique combination of functional groups in AMP2ol suggests potential applications in various fields:
1. Pharmaceutical and Medicinal Chemistry
The presence of both amino and hydroxyl groups makes AMP2ol a potential building block for synthesizing biologically active molecules. It could serve as a precursor for:
-
Drug Intermediates: Its chiral nature makes it potentially useful in the synthesis of chiral drugs, where stereochemistry plays a crucial role in biological activity and efficacy.
-
Ligands for Metal Complexes: The amino and hydroxyl groups can coordinate with metal ions, potentially leading to the development of metal-based drugs or catalysts.
2. Material Science
AMP2ol's properties might find applications in material science:
-
Polymer Chemistry: It could be incorporated into polymer chains to impart specific properties, such as improved biocompatibility or enhanced interaction with other materials.
-
Surfactants and Emulsifiers: The amphiphilic nature of AMP2ol (possessing both hydrophilic and hydrophobic regions) might make it suitable as a surfactant or emulsifier in various applications.
3. Catalysis
The chiral nature of AMP2ol could be exploited in asymmetric catalysis:
- Chiral Ligands: It could serve as a ligand in transition metal-catalyzed reactions, leading to enantioselective synthesis of valuable chiral compounds.
Synthesis of 2-Amino-4-methylpentan-2-ol
Several synthetic routes could be employed to produce AMP2ol. The specific choice depends on factors such as desired enantiomeric purity, cost-effectiveness, and scalability. Potential strategies include:
-
Reductive Amination: This approach could involve the reductive amination of 4-methylpentan-2-one using ammonia or a suitable amine source, followed by reduction of the resulting imine.
-
Grignard Reaction: A suitable Grignard reagent could react with a chiral epoxide derivative, followed by amination.
-
Asymmetric Synthesis: Enantioselective synthesis could be achieved using chiral catalysts or reagents to control the stereochemistry of the product. This is particularly important if a specific enantiomer is required for a specific application.
Safety Considerations and Handling
As with any chemical compound, appropriate safety precautions should be taken when handling AMP2ol. Specific hazards and handling procedures need to be determined through detailed safety assessments. General precautions include:
-
Protective Equipment: Appropriate personal protective equipment (PPE), such as gloves, safety goggles, and lab coats, should be worn.
-
Ventilation: Adequate ventilation should be ensured to minimize exposure to potential vapors.
-
Disposal: Proper disposal procedures should be followed in accordance with local regulations.
-
Toxicity: The toxicity of AMP2ol requires thorough investigation. Studies on acute and chronic toxicity, as well as potential mutagenic or carcinogenic effects, are crucial before any large-scale application.
Future Research Directions
Further research is needed to fully understand the potential of AMP2ol. Areas requiring further investigation include:
-
Detailed biological activity studies: Exploring its potential interactions with biological systems and receptors.
-
Optimization of synthetic routes: Developing more efficient and cost-effective methods for its synthesis.
-
Exploration of novel applications: Investigating its potential use in various fields beyond those currently considered.
-
Comprehensive toxicology studies: A thorough investigation of its safety profile is crucial before widespread application.
This comprehensive overview provides a foundation for understanding 2-Amino-4-methylpentan-2-ol. Its unique combination of functional groups and chiral nature suggests considerable potential across various scientific and technological domains. However, further research and development are necessary to fully realize this potential and ensure its safe and responsible use. The information presented here serves as a starting point for future explorations and innovations centered around this interesting molecule.
Latest Posts
Latest Posts
-
What Is 5 Of 90
Sep 25, 2025
-
Wxy Is A Right Triangle
Sep 25, 2025
-
Standing Up To Absolute Power
Sep 25, 2025
-
Globalization Has Caused Intercultural Transients
Sep 25, 2025
-
What Are Machines Fueled By
Sep 25, 2025
Related Post
Thank you for visiting our website which covers about Ch3ch Nh2 Ch2ch Ch3 Oh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.