Molar Mass Of Glucose C6h12o6
gasmanvison
Sep 12, 2025 · 6 min read
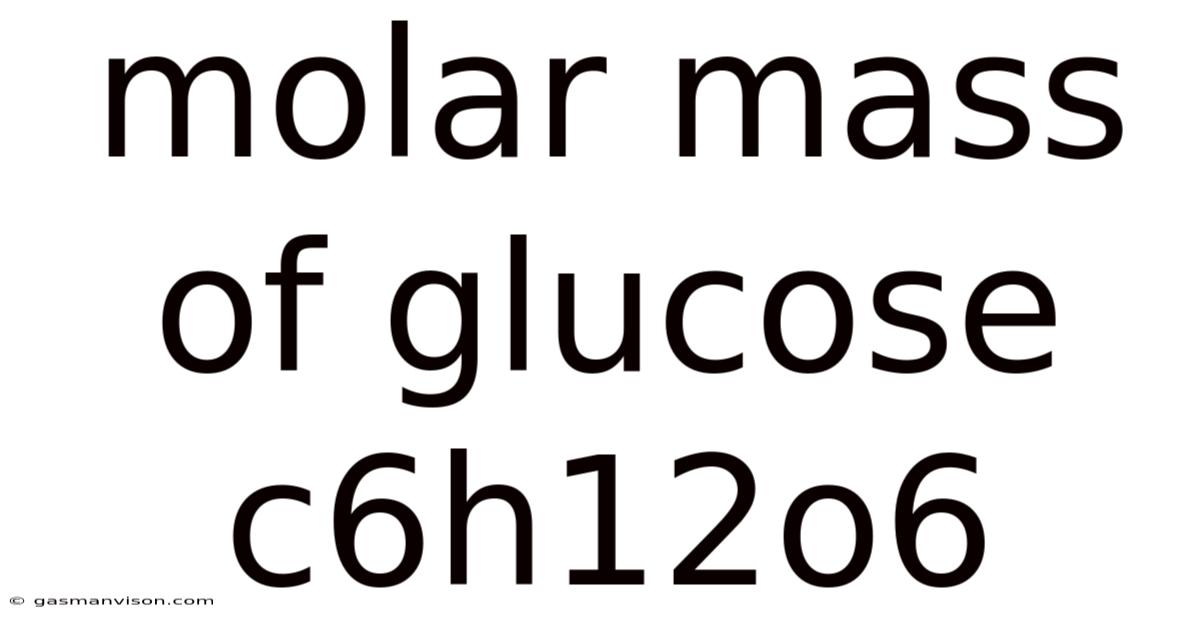
Table of Contents
Understanding the Molar Mass of Glucose (C₆H₁₂O₆): A Comprehensive Guide
Meta Description: This comprehensive guide explores the molar mass of glucose (C₆H₁₂O₆), explaining its calculation, significance in chemistry and biology, and its applications in various fields. Learn about molar mass, molecular weight, and their practical uses.
Glucose, a simple sugar with the chemical formula C₆H₁₂O₆, plays a crucial role in biological systems as the primary source of energy for living organisms. Understanding its molar mass is fundamental to various scientific disciplines, from biochemistry and medicine to food science and industrial chemistry. This article provides a detailed explanation of the molar mass of glucose, its calculation, significance, and applications. We'll delve into the concept of molar mass itself, clarifying its relationship to molecular weight and how it's used in stoichiometric calculations.
What is Molar Mass?
Molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of elementary entities, such as atoms, molecules, or ions. Therefore, the molar mass expresses the mass of 6.022 x 10²³ molecules of a given substance, typically expressed in grams per mole (g/mol). It's a crucial concept for converting between the mass of a substance and the number of moles present, a critical step in many chemical calculations. Often, the terms molar mass and molecular weight are used interchangeably, although technically they represent slightly different concepts. Molecular weight is the sum of the atomic weights of the atoms in a molecule, while molar mass is the mass of one mole of that molecule. In practice, the numerical values are essentially the same.
Calculating the Molar Mass of Glucose (C₆H₁₂O₆)
Calculating the molar mass of glucose involves summing the atomic masses of its constituent atoms: six carbon atoms (C), twelve hydrogen atoms (H), and six oxygen atoms (O). We use the standard atomic weights from the periodic table:
- Carbon (C): Approximately 12.01 g/mol
- Hydrogen (H): Approximately 1.01 g/mol
- Oxygen (O): Approximately 16.00 g/mol
Therefore, the molar mass of glucose (C₆H₁₂O₆) is calculated as follows:
(6 x 12.01 g/mol) + (12 x 1.01 g/mol) + (6 x 16.00 g/mol) = 180.18 g/mol
This means that one mole of glucose weighs approximately 180.18 grams. This value is essential for various chemical and biological calculations, allowing scientists to convert between the mass of glucose and the number of moles present.
Significance of Molar Mass in Chemistry and Biology
The molar mass of glucose has significant implications across various scientific fields:
-
Stoichiometry: Molar mass is crucial for stoichiometric calculations, allowing scientists to determine the amounts of reactants and products involved in chemical reactions. For example, knowing the molar mass of glucose allows us to calculate the amount of oxygen required for its complete combustion or the amount of carbon dioxide and water produced.
-
Solution Preparation: In biochemistry and analytical chemistry, accurate molar mass values are vital for preparing solutions of known concentrations. Researchers often need to prepare glucose solutions of specific molarity (moles per liter) for experiments or assays. Using the molar mass, they can precisely weigh out the required amount of glucose to achieve the desired concentration.
-
Metabolic Processes: In biology, understanding the molar mass of glucose is crucial for studying metabolic processes. Metabolic pathways, such as glycolysis and cellular respiration, involve precise quantities of glucose and other metabolites. Knowing the molar mass allows researchers to quantify the amounts of glucose involved in these processes and study their kinetics.
-
Food Science and Nutrition: In food science and nutrition, molar mass is used to determine the energy content of foods. Glucose is a primary source of energy, and its molar mass is used to calculate the energy yield from its metabolism (approximately 4 kcal/gram).
-
Pharmaceutical Applications: Many pharmaceutical formulations utilize glucose or glucose derivatives. Accurate molar mass determination is essential for ensuring the correct dosage and efficacy of these medications.
Applications of Glucose and its Molar Mass
The applications of glucose and the knowledge of its molar mass are widespread:
-
Energy Production: Glucose is the primary fuel for cellular respiration, the process that generates ATP, the cell's main energy currency. Understanding its molar mass is crucial for studying energy metabolism.
-
Biosynthesis: Glucose is a precursor for the synthesis of many essential biomolecules, including glycogen (energy storage in animals), starch (energy storage in plants), and cellulose (structural component of plants). The molar mass plays a role in understanding the stoichiometry of these biosynthetic pathways.
-
Medical Diagnostics: Blood glucose levels are a critical indicator of health. Measurements are often expressed in mg/dL (milligrams per deciliter) and can be converted to molar concentration using the molar mass of glucose.
-
Industrial Applications: Glucose is used in various industrial processes, including the production of ethanol (biofuel), various food products, and pharmaceuticals. Accurate knowledge of its molar mass is important for process optimization and quality control.
-
Research and Development: Glucose and its derivatives are used extensively in research and development, particularly in fields like biochemistry, cell biology, and drug discovery. Molar mass is a key parameter for many experiments.
Beyond the Basics: Isotopes and Molar Mass
The molar mass calculated above (180.18 g/mol) uses the average atomic masses of carbon, hydrogen, and oxygen. These average atomic masses account for the natural abundance of different isotopes of each element. Isotopes are atoms of the same element with differing numbers of neutrons. For example, carbon exists as ¹²C, ¹³C, and ¹⁴C, each with a slightly different mass. The average atomic mass reflects the weighted average of these isotopes' masses based on their natural abundance. If you were working with a sample of glucose enriched in a specific isotope (e.g., a sample with a higher proportion of ¹³C), the molar mass would be slightly different. This subtle variation is typically negligible for most applications, but it's important to understand that the 180.18 g/mol value is an average.
Practical Considerations and Error Analysis
When working with molar mass in real-world experiments, several factors can introduce errors:
-
Measurement Accuracy: Precise weighing of glucose is crucial for accurate molarity calculations. Errors in weighing can propagate through subsequent calculations, affecting the final results.
-
Purity of the Sample: Impurities in the glucose sample can affect the accuracy of molar mass-based calculations. A sample containing other substances will lead to an incorrect molar mass determination.
-
Temperature and Pressure: For gases, temperature and pressure influence volume and density, which in turn affect molar mass determinations. Accurate temperature and pressure measurements are crucial for accurate results.
-
Instrumental Errors: Errors associated with analytical instruments (e.g., balances, spectrometers) used to determine molar mass must be considered.
Conclusion
The molar mass of glucose (C₆H₁₂O₆), approximately 180.18 g/mol, is a fundamental value in chemistry and biology. Its accurate determination is crucial for numerous applications, ranging from stoichiometric calculations and solution preparation to metabolic studies and industrial processes. Understanding the concept of molar mass, its calculation, and its significance allows for a deeper comprehension of the role glucose plays in various scientific disciplines. While the average molar mass is sufficient for most applications, remembering the influence of isotopes and potential experimental errors is important for precise work. By accurately determining and utilizing the molar mass of glucose, scientists can gain valuable insights into its behavior and its importance in biological and chemical systems.
Latest Posts
Latest Posts
-
What Do Heb Stand For
Sep 13, 2025
-
Number Of Protons In Mg
Sep 13, 2025
-
How Many Lbs Is 57kg
Sep 13, 2025
-
50 Ml How Many Oz
Sep 13, 2025
-
14 5 Pinned And Welded Upper
Sep 13, 2025
Related Post
Thank you for visiting our website which covers about Molar Mass Of Glucose C6h12o6 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.